Millions of people are taking anti-inflammatory medications. Most of them fail to recognize that the inflammatory process is nature's chief method of healing. This does not mean that reducing or eliminating inflammation does not have its place, but thank goodness for the inflammatory process.
 Patella tendinosis and epicondylopathy have been treated by creation of an inflammatory process.2,3 In these cases, the use of ultrasound to determine the location of the pathological tissue is accompanied by dry needling. Next, autologous (the patient's) blood is injected into the lanced areas of the abnormal tendon. The dry needling is used to incite internal hemorrhage, resulting in an inflammatory response to "induce the formation of granulation tissue, which strengthens the tendon."
Patella tendinosis and epicondylopathy have been treated by creation of an inflammatory process.2,3 In these cases, the use of ultrasound to determine the location of the pathological tissue is accompanied by dry needling. Next, autologous (the patient's) blood is injected into the lanced areas of the abnormal tendon. The dry needling is used to incite internal hemorrhage, resulting in an inflammatory response to "induce the formation of granulation tissue, which strengthens the tendon."
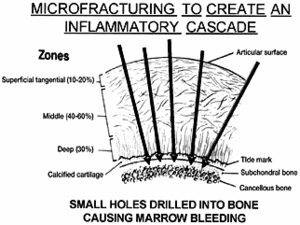
Cartilage, for example, has no blood, nerve or lymphatic supply when injured. Although the body will attempt to remodel by metabolizing some chondrocytes, they are incapable of producing the required repair products.4 The fibrous tissue that appears contains type I collagen, which is associated with bone, tendons and ligaments, instead of the type II that is responsible for hyaline cartilage. So, how can circulation be produced to stimulate healing and possibly the production of new cartilage? Since inflammation requires circulation, one method to stimulate vascularity is to penetrate the bone beneath it (subchondral plate), which has a plentiful amount of vascular elements.
A technique called microfracture is now being used for focal traumatic chondral (cartilage) defects, as well as for degenerative lesions.4 In this method, the calcified cartilage layer is first debrided very carefully, so as not to violate the subchondral bone. Then multiple small holes are made into the bone and spaced 1-2 mm apart, causing marrow bleeding to occur, flowing from the small holes to fill the chondral defect. A rough surface is created for adherence of the blood clot, containing the undifferentiated mesenchymal cells derived from the subchondral bone. There is some debate on the end results, regarding new hyaline cartilage. Some researchers state that it converts to a hyaline-like chondroid tissue. Other studies show a shift from type I to hyaline-cartilage type II collagen within five to six weeks. While there may be up to 20 percent of type I remaining, the use of continuous passive motion is reported to increase the amount of type II collagen.5
In both, the methods of creating inflammation above the typical phases of necrosis, inflammation and repair occur. For example, Graston technique initiates the inflammatory process by introducing a small amount of trauma to the affected area. Re-inflammation triggers the healing cascade by enhancing the proliferative invasion of blood, nutrients and fibroblasts to the region, which results in collagen deposition and maturation.6 In the methods described above, it has been hypothesized that basic fibroblast growth factor and transforming growth factor may act as humoral mediators in the induction of the healing cascade.7
References
- Mankin HJ. The response of articular cartilage to mechanical injury. J Bone & Joint Surg, 1982;64-A(3):460-6.
- Edwards SG, Calandruccio JH. Autologous blood injections for refractory lateral epicondylitis. J Hand Surg [Am], March 2003;28(2):272-8.
- James SL, Ali K, Pocock C, et al. Ultrasound guided dry needling and autologous blood injection for patella tendinosis. Br J Sports Med, Mar 26, 2007 [E-pub ahead of print].
- Gill TJ, Asnis PD, Berkson EM. The treatment of articular cartilage defects using the microfracture technique. J Orthop Sports Phys Ther, 2006;36(10):728-38.
- Salter RB. The biologic concept of continuous passive motion of synovial joints. The first 18 years of basic research and its clinical application. Clin Orthop Relat Res, 1989;12-25.
- Carey-Loghmani MT, Hammer WI. Graston technique. In: Hammer W. Functional Soft-Tissue Examination and Treatment by Manual Methods, 3rd ed. Sudbury, Mass.: Jones & Bartlett, 2007, pp. 589-625.
- Iwasaki M, Nakahara H, Nakata K, et al. Regulation of proliferation and osteochondrogenic differentiation of periosteum-derived cells by transforming growth factor-and basic fibroblast growth factor. J Bone Joint Surg Am, 1995;77:543-54.
Click here for previous articles by Warren Hammer, MS, DC, DABCO.





