Spine stability is known to depend on agonist-antagonist muscle coactivation. This involuntary motor control is guided by central nervous system programs which develop in early infancy. New avenues of musculoskeletal treatment are being explored that have their roots in developmental kinesiology.16,17
Agonist-Antagonist Muscle Imbalance
The search for patterns of how individual muscles react to injury or inflammation has continued for many decades.
A commonly held theory to explain muscle dysfunction is the pain-spasm-pain model. However, this overstates the importance of muscle tension and does not account for muscle inhibition. As the evidence suggests, muscle imbalance is the rule, with certain muscles tending toward hyperactivity and others toward inhibition. Lund proposed the pain-adaptation theory to explain such imbalances.18 He hypothesized that when pain is present, there is a decreased activation of muscles during movements in which they act as agonists and increased activation during movements in which they are antagonists. Preliminary tests of this theory have reported that overactivity of antagonist back muscles during the ipsilateral swing phase of gait, and decreased agonist peak muscle activity during the double stance phase, distinguish chronic LBP sufferers from controls.1 Other research has shown that if saline is injected into the lower leg muscles, gait becomes dyscoordinated, with a decrease in EMG activity in agonist muscles and increase in EMG activity of antagonists.7
The Neurodevelopmental Basis for Muscle Imbalance
Janda suggested there is a group of "postural" muscles (i.e., gastrocnemius, upper trapezius, SCM, erector spinae) that are involved in static tasks, such as standing or sitting, and that have a tendency to become overactive.11,12,13 Another group is termed "phasic" because the muscles are involved in producing dynamic movements such as head flexion, arm elevation or trunk curling (i.e., deep neck flexors, inferior scapular fixators, abdominals) and tend to become inhibited. This notion emerged from his observation among individuals with neurologic diseases that spasticity (e.g., cerebral palsy) usually favored certain muscles (i.e., extremity flexors, adductors and internal rotators) and paralysis (e.g., stroke) of other muscles (i.e., extremity extensors, abductors and external rotators).
Janda described how the postural muscle system was needed for preservation of basic functions and that its muscles were usually spared, while those required for more dynamic activities were more fragile and easily compromised. He also noted that these same tendencies were seen in individuals without neurological disease, who either remained constrained sitting postures for prolonged periods of time) or were training their muscles inappropriately. This concept of muscle imbalance came under scientific scrutiny with Bergmark,4 who proposed that muscles could be classified broadly as deep, local stabilization muscles or superficial, global mobilization muscles.
Not surprisingly, the muscle imbalance theory has a neurodevelopmental basis. In the young infant (less than 1 month old), the fetal position is maintained by "tonic" contraction of trunk and extremity flexors, along with extremity adductors and internal rotators. Reciprocal inhibition (Sherrington's law), which is present in early infancy, inhibits the antagonists of the "tonic" muscle chains. As the infant develops, reciprocal inhibition becomes dampened, thus allowing the "phasic" muscle system to activate - this process fails in cerebral palsy. As the "reflex bound" infant begins to develop a postural control system, the "tonic" activity of muscles, which maintains the fetal posture, is superceded by agonist-antagonist coactivation of muscles necessary for movement control. Thus, extensors, abductors, and external rotators coactivate with their fetal partners to stabilize joints and allow for the neurodevelopment of upright posture.
For instance, orientation movements of the head and neck begin between 4 and 6 weeks as of age the deep cervical flexors activate to coordinate movement with the cervical extensors.27 Similarly, the inferior scapular fixators begin to activate in the following months to balance the activity of the upper trapezius and levator scapulae and allow for scapulothoracic stabilization during arm movements (grasping, prehension, pushing, pulling, etc.).
A typical example of a failure of this agonist-antagonist coactivation occurs in Sprengel's deformity, whereby the scapula fails to descend.
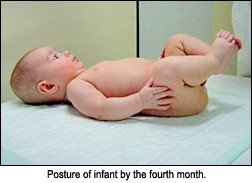 During neurodevelopment, the infant learns to support his or herself using different points of support, which facilitates a wide variety of postures and movements. The ensuing muscle coactivation centrates joints in a position of maximum congruence of joint surfaces. This allows for maximum load-bearing potential and thus facilitates further functional development of the infant. The first sign of this is in the head and neck by the end of the first month of life, and progresses in the sagittal plane until the infant has assumed a full squat position - although not weight-bearing - by the end of the fourth month of life (see image). Between the fourth and sixth months of life, oblique muscles begin to work together which cross the midline and allow for creeping, crawling and turning over. Neurodevelopment has achieved its pinnacle when, sometime in the third year, the child is able to balance on one leg while simultaneously abducting and externally rotating the shoulders 90 degrees.
During neurodevelopment, the infant learns to support his or herself using different points of support, which facilitates a wide variety of postures and movements. The ensuing muscle coactivation centrates joints in a position of maximum congruence of joint surfaces. This allows for maximum load-bearing potential and thus facilitates further functional development of the infant. The first sign of this is in the head and neck by the end of the first month of life, and progresses in the sagittal plane until the infant has assumed a full squat position - although not weight-bearing - by the end of the fourth month of life (see image). Between the fourth and sixth months of life, oblique muscles begin to work together which cross the midline and allow for creeping, crawling and turning over. Neurodevelopment has achieved its pinnacle when, sometime in the third year, the child is able to balance on one leg while simultaneously abducting and externally rotating the shoulders 90 degrees.
Vojta, who developed a rehabilitation system for treatment of neurological diseases such as cerebral palsy, stroke, and spinal cord injury, formulated the idea that the fetal muscles were more primitive, and thus, better insulated, whereas the muscles needed for development of the upright posture were younger phylogenetically and thus more fragile biologically.27 Preliminary investigations suggest imbalances between these systems of muscles may be present in 30 percent of young children without neurological disease.27
Clinical Implications
Children and adults in modern society spend a great deal of time sitting. The slumped sitting posture brings about changes in lumbopelvic, thoracic, and head and neck posture and function. In particular, the muscles associated with the fetal position will be maintained in shortened lengths while their antagonists will be lengthened. The resulting lumbar kyphosis during bending tasks and round shouldered/head forward posture predisposes people toward musculoskeletal problems.
Muscle imbalance typically alters the performance of movement patterns - including activities of daily living - with the ultimate result being increased joint instability. Janda has described the typical patterns of muscle imbalance as the lower crossed syndrome and upper crossed syndrome.13
Kolar has shown how reflex activation of the diaphragm via methods integrating current spine stabilization techniques with Vojta's techniques of reflex locomotion can be utilized to improve agonist-antagonist muscle coactivation patterns and thus stability.16,17
References
- Arendt-Nielson L, Graven-Nielson T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait Pain 1995;64:231-240.
- Balster SM, Jull GA. Upper trapezius muscle activity during the brachial plexus tension test in asymptomatic subjects Man Ther 1997;2:144-149.
- Bansevicius D, Sjaastad O. Cervicogenic headache: the influence of mental load on pain level and EMG of shoulder-neck and facial muscles Headache 1996;36:372-378.
- Bergmark A. Stability of the lumbar spine. A study in mechanical engineering Acta Orthopedica Scandinavica 1989;230:20-24.
- DeAndrade JR, Grant C, Dixon A St. J. Joint distension and reflex muscle inhibition in the knee J. Bone Joint Surg 1965;47: 313-322.
- Floyd WF, Silver PHS. Function of the erector spinae muscles in certain movements and postures in man J Physiol 1955;129:184-203.
- Graven-Nielsen T, Svensson P, Arendt-Nielsen L. Effects of experimental muscle pain on muscle activity and co-ordination during static and dynamic motor function Electroencephalogr Clin Neurophysiol 1997;105:156-164.
- Hall T, Quintner J. Responses to mechanical stimulation of the upper limb in painful cervical radiculopathy. Aust J Physiotherapy 1996;42:277-285.
- Hallgren R, Greenman P, Rechtien J. Atrophy of suboccipital muscles in patients with chronic pain: a pilot study. J Am Osteopath Assoc 1994;94:1032-1038.
- Hides JA, Stokes MJ, Saide M, Jull GA, Cooper DH. Evidence of lumbar multifidus muscle wasting ipsilateral to symptoms in patients with acute/subacute low back pain. Spine 1994;19:165-172.
- Janda V. On the concept of postural muscles and posture in man. Aus J Physioth 1983;29:83-84.
- Janda V. Muscles, Central Nervous Motor Regulation and Back Problems. In: Korr IM (editor): The Neurobiologic Mechanisms in Manipulative Therapy. Plenium Press, New York, pp. 27-41, 1978.
- Janda V. Physical Examination of the Muscular System in Rehabilitation of the Spine: A Practitioners Manual. Liebenson C (editor). Lippincott/Williams and Wilkins, Baltimore, 2006.
- Jull GA. Deep cervical flexor muscle dysfunction in whiplash. Journal of Musculoskeletal Pain 2000;8:143-154.
- Jull G, Barret C, Magee R, Ho P. Further clinical clarification of the muscle dysfunction in cervical headache. Cephalgia 1999;19:179-185.
- Kolár P. The sensomotor nature of postural functions, its fundamental role in Rehabilitation. Orthopedic Medicine 1999;21(2):40-45.
- Kolár P. Facilitation of Agonist-Antagonist Co-Activation by Reflex Stimulation Methods in Rehabilitation of the Spine: A Practitioners Manual. Liebenson C (editor). Lippincott/Williams and Wilkins, Baltimore, 2006.
- Lund JP, Donga R, Widmer CG, et al. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol 1991;69:683-94.
- Madeleine P, Lundager B, Voight M, Arendt-Nielsen L. Shoulder muscle co-ordination during chronic and acute experimental neck-shoulder pain: an occupational pain study. Eur J Appl Physiol 1999;79:127-140.
- Mannion AF, Taimela S, Muntener M, Dvorak J. Active therapy for chronic low back pain. Part 1. Effects on back muscle activation, fatigability, and strength. Spine 2001;26:897-908.
- McPartland JM, Brodeur RR, Hallgren RC. Chronic neck pain, standing balance and suboccipital muscle atrophy: a pilot study. J Manipulative Physiol Ther 1997;20:24-29.
- Nederhand MJ, Ijzerman MJ, Hermens HK, et al. Cervical muscle dysfunction in the chronic whiplash associated disorder grade II (WAD-II). Spine 2000;15;1938-1943.
- Shirado O, Ito T, Kaneda K, et al. Flexion-relaxation phenomenon in the back muscles: a comparative study between healthy subjects and patients with chronic low back pain. Am J Phys Med Rehabili 1995;74:139-144.
- Spencer JD, Hayes KC and Alexander IJ. Knee joint effusion and quadriceps reflex inhibition in man. Arch Phys Med Rehab 1984;65:171-177.
- Stokes M, Young A. Investigations of quadriceps inhibition: implications for clinical practice. Physiotherapy 1984;70:425-428.
- Triano JJ, Schultz AB. Correlation of objective measure of trunk motion and muscle functiona with lo-back disability ratings. Spine 1987;12:561-5.
- Vojta V, Peters A. Das Vojta Princip. Heidelberg, Springer-Verlag, 1992.
- Yoshihara K, Shirai Y, Nakayama Y, Uesaka S. Histochemical changes in the multifidus muscle in patients with lumbar intervertebral disc herniation. Spine 2001;26:622-626.
- Zhao WP, Kawaguchi Y, Matsui H, Kanamori M, Kimura T. Histochemistry and morphology of the multifidus muscle in lumbar disc herniation. Comparative study between diseased and normal sides. Spine 2000;25:2191-2199.
Click here for previous articles by Craig Liebenson, DC.





