Author's note: The following is an excerpt from a chapter I was asked to write for a new law book.
In spinal trauma or disease, the neurological exam chiefly aims to determine whether one (or more) of three basic neurological conditions is present: myelopathy, radiculopathy and peripheral nerve disorder. Myelopathy is a general term for a disorder affecting the spinal cord itself. When the nerve roots (radicles) themselves are injured or compressed, we call that condition radiculopathy.
The spinal cord is part of the central nervous system (CNS), so a myelopathy is considered a CNS disorder. The nerve root and the peripheral nerves they become are regarded as the peripheral nervous system. Let's review basic elements of the neurological examination in the context of the meaning of some findings and what we might derive from them; and address some of the misconceptions that remain prevalent today. Let's start by considering just myelopathy and radiculopathy for a moment.
Reflex Testing
In myelopathy, patients may display what are termed long track signs (or upper motor neuron lesions), which often manifest as weakness and clumsiness and unsteady gait. Examination may reveal increased (i.e., more brisk) deep tendon reflexes (DTR). In more severe cases, clonus or rhythmic involuntary contractions may result when the foot is forcefully dorsiflexed by the examiner. And although dozens of pathological reflexes have been described over the years, the most commonly relied upon today are Hoffman's sign in the hand and Babinski's sign in the foot. When present, they are indicative of myelopathy.
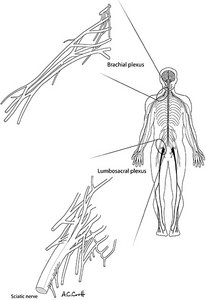 Figure 1. The human nervous system. The important nerve roots emanating from the cervical spine coalesce into the brachial plexus before becoming named peripheral nerves. Those of the lumbosacral spine coalesce into the lumbosacral plexus before forming the named peripheral nerves such as the sciatic nerve. [Used with permission from Whiplash and Mild Traumatic Brain Injuries (2009).]
Visualizing the brain as an electric motor and the spinal cord as a conduit of all of the wires going to all muscles (machines), it's easy to imagine how an injury to the cervical spine might only affect the upper extremities; or that it could affect the lower extremities as well. (Figure 1) In a complete cord transection, of course, all neurological function below the level of the lesion will be lost. If this happens in the upper cervical spine, the patient will be left quadriplegic.
Figure 1. The human nervous system. The important nerve roots emanating from the cervical spine coalesce into the brachial plexus before becoming named peripheral nerves. Those of the lumbosacral spine coalesce into the lumbosacral plexus before forming the named peripheral nerves such as the sciatic nerve. [Used with permission from Whiplash and Mild Traumatic Brain Injuries (2009).]
Visualizing the brain as an electric motor and the spinal cord as a conduit of all of the wires going to all muscles (machines), it's easy to imagine how an injury to the cervical spine might only affect the upper extremities; or that it could affect the lower extremities as well. (Figure 1) In a complete cord transection, of course, all neurological function below the level of the lesion will be lost. If this happens in the upper cervical spine, the patient will be left quadriplegic.
Every part of the nervous system external to the spinal cord (with the exception of the autonomic nervous system) is considered the peripheral nervous system and lesions associated with it are referred to as lower motor neuron lesions. In a radiculopathy, the DTRs, if they are abnormal at all, will be diminished. And, when substantial motor fibers become involved, the muscles innervated by the affected root will become weak and relatively flaccid, rather than spastic as with a myelopathy. However, very small bundles of muscle fibers may fire spontaneously when denervated – a phenomenon called fasciculation.
In the case of cervical spine trauma or disease, it is possible to have both a myelopathy and a radiculopathy. For example, a disc may herniate rearward into the cord and laterally to compress the adjacent nerve root.
Muscle Testing
Doctors evaluate muscle strength for a number of reasons. As a paramedic at the scene of a trauma or in the ER, I was concerned only that a spinal cord injury or stroke might have occurred. Later, as a chiropractor, I was more interested in the function of specific muscles or groups of muscles. But manual muscle tests are crude and are graded on an ordinate scale. In a spinal trauma rehabilitation center, we grade muscular strength on a 0-5 scale as follows:
- Grade 0 = no evidence of contraction
- Grade 1 = some contraction, but no movement against gravity
- Grade 2 = movement of joint with gravity removed
- Grade 3 = movement against gravity, but not against resistance
- Grade 4 = movement against partial resistance, but still with significant weakness
- Grade 5 = normal strength against maximal resistance
Clearly there is an element of art involved. Experiments have demonstrated that even trained and experienced examiners, when using manual methods (i.e., their hands) to test muscle strength, could not reliably detect weakness in patients until the weaknesses approached 30-40 percent.1 Conversely, using computerized isotonic dynamometry, it is claimed that weaknesses of as little as 10 percent or so can be reliably detected.2
In the medicolegal setting, muscle strength has historically been used to either verify or refute impairment. But the potential for examiner bias, coupled with the poor sensitivity of manual muscle testing, would suggest a modicum of caution be exercised in the interpretation of such findings.
Sensory Testing
Although the sensory examination is most often conducted using the familiar pinwheel, different kinds of sensory nerves respond to other types of stimulation. In addition to pain, for example, we also perceive temperature, light touch and deep pressure. These can be tested using hot or cold items, wisps of cotton, and the application of pressure.
The perception of two point discrimination is an important facility measured by determining how far apart two distinct points on a patient's skin surface must be to enable the patient to perceive the two distinct points. This should be a matter of a few millimeters on the fingertips, but may be as much as a few inches on the back.
Other parts of the nervous system convey the important sense of proprioception, which allows us to be able to sense the position of our joints and our bodies in space, even with our eyes closed. One can bend the great toe in different positions and ask the patient to describe them spatially. Proprioceptive disorders can occur in myelopathy, such as from spinal stenosis, and adversely affect gait and balance.
In a previous column, I discussed the so-called non-organic signs and, in particular, those controversial Waddell's signs. I suggested it is time to relegate them to the scrapheap of unverifiable science. But just as we have placed too much faith in Waddell's signs, we often read too much into sensory testing.
 Figure 2. The standard dermatome map of sensory innervation. Individual dermatomes are not labeled here. [Used with permission from Whiplash and Mild Traumatic Brain Injuries (2009).]
The body surface has been mapped out into discrete sections known as dermatomes. These represent the approximatedistributions of each of the spinal nerve roots. First mapped in the late 19th century, these maps are relied upon, along with other tests, in the planning of surgery or other procedures in patients suffering from peripheral neuropathy. In the forensic setting they are also used as an indicator of malingering. The term of art for what are felt to be unexpected or non-dermatomal findings is non-organic signs. But is this likely to be true?
Figure 2. The standard dermatome map of sensory innervation. Individual dermatomes are not labeled here. [Used with permission from Whiplash and Mild Traumatic Brain Injuries (2009).]
The body surface has been mapped out into discrete sections known as dermatomes. These represent the approximatedistributions of each of the spinal nerve roots. First mapped in the late 19th century, these maps are relied upon, along with other tests, in the planning of surgery or other procedures in patients suffering from peripheral neuropathy. In the forensic setting they are also used as an indicator of malingering. The term of art for what are felt to be unexpected or non-dermatomal findings is non-organic signs. But is this likely to be true?
Importantly, the original dermatome maps were derived by mapping the loss of sensation caused by disc herniations. These maps were deliberately made discrete and non-overlapping in order to simplify them make them more utilitarian. (Figure 2) Think AMA Guidelines: generally lacking in both construct and criterion validity.3
Experiments with primates has shown, however, that even a complete severance of a nerve root will not leave any area of skin without sensation, so there is, in contrast to these artificially discretized dermatome maps, a considerable degree of overlap in sensory distribution. And a more recent study correlating the distribution of pain or paresthesia arising from root irritation in humans revealed that it is not uncommon for a patient with a focal root lesion to describe seemingly non-dermatomal symptoms.4
It's also been demonstrated that persons with transitional lumbar vertebrae will display altered dermatomal patterns.5,6 And there are still other explanations of non-dermatomal findings.
During the early years of World War II, some classic experiments were conducted in Great Britain by an sedulous worker destined for great fame, Jonas Kellgren.7 I actually had the pleasure of dining with one of his research colleagues many years ago. In these experiments, it was demonstrated that by merely irritating paraspinal tissue, such as the covering of bone (periosteum), joint capsules, etc., with a minute injection of irritant or mechanical scratching, they could produce radiating pain and paresthesiae (e.g., numbness and tingling) similar to the kinds of radiating symptoms seen in radiculopathy. And yet, it was not quite the same in terms of its distribution.
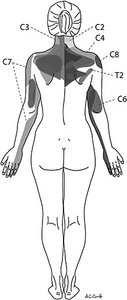 Figure 3. Segmental map of referred pain and paresthesia produced by irritation of paraspinal tissues, rather than nerve roots. [Used with permission from Whiplash and Mild Traumatic Brain Injuries (2009).]
In contrast to the orderly and fairly discrete dermatomal patterns representing peripheral nerves, these sclerotomal patterns, as they were initially called, were overlapping and less organized. (Figure 3) Yet they were reproducible across a number of volunteers. That research lay fallow for some years until other groups in the 1940s and 1950s expanded upon it.8-9 As recently as the 1990s, it has been revisited by Nick Bogduk and company.10-11
Figure 3. Segmental map of referred pain and paresthesia produced by irritation of paraspinal tissues, rather than nerve roots. [Used with permission from Whiplash and Mild Traumatic Brain Injuries (2009).]
In contrast to the orderly and fairly discrete dermatomal patterns representing peripheral nerves, these sclerotomal patterns, as they were initially called, were overlapping and less organized. (Figure 3) Yet they were reproducible across a number of volunteers. That research lay fallow for some years until other groups in the 1940s and 1950s expanded upon it.8-9 As recently as the 1990s, it has been revisited by Nick Bogduk and company.10-11
Years ago, I chaired a breakout meeting of the World Congress of Pain on this subject. Susan Lord, a co-worker of Bogduk's whose work has centered on pain management and who has become famous for the placebo-controlled studies which identified the facet joint as a major pain generator after whiplash,12 joined me. As it turned out, we had one of the largest attendances of the breakout sessions, with standing room only; which is insightful in and of itself.
In spite of the prevailing belief in medicine that pain follows only the well-charted pathway found in textbooks, there are many who are embracing more complex models of pain today. One of the attendees at that World Congress of Pain, who came up and spoke with me on this subject for some time afterward, was David Simons, whose classic work with Janet Travell has long served as the Bible of myofascitis diagnosis and management.13
Complicating matters, I think it is quite common that patients who have had a more severe form of spinal trauma will simultaneously display signs of any combination of referred pain, discogenic pain and/or neurologically mediated pain. In my forensic practice, I routinely see this – not that I claim to be able to untangle the melange of complaints, symptom by symptom.
Nevertheless, these patients do get some temporary relief after diagnostic nerve root blocks, some relief after medial branch blocks, and some after ESI, while discography can often reproduce concordant pain at one or more levels. This is precisely why surgery so often fails to give complete relief in this group of patients: it can never be the global remedy surgeons hope it will be because there are frequently multiple levels of injury involving multiple types of soft tissue: discs, ligaments, facet joint capsules and others.
There is great interest in what we now call referred pain from clinicians working in the pain-management field. It has spawned new facets of pain diagnostics such as the medial branch block (MBB), various other local anaesthetic blocks, and epidural steroid injections (ESI); and revivified some older ones like discography.
Meanwhile, among many of the rank and file in orthopaedics and neurology, radiating arm or leg pain is nearly always taken to be some manifestation of radiculopathy or peripheral neuropathy. And when these conditions are ruled out, the alternative explanations often turn to the non-organic including secondary gain or psychological states. (In a previous column, I also discussed what used to be termed somatoform disorder, which is a distant cousin of secondary gain.)
A popular explanation for irregular or non-neurogenic pain in the world of psychology these days is the so-called lack of "coping skills," which tends to discount the chronic pain patient for failing to effectively manage their life. Like most of psychology, a lot of begging of the question necessarily takes place: How much pain does the patient actually have and how well would the average person be expected to cope with it?
A solid scientific approach to the question of construct validity is nebulous at best. I suspect that the real utility of such papers is either as a lever for those wishing to reduce their exposure in litigated cases or as a convenient subject for a master's thesis for psychology graduate students.
So, from research going as far back as the 1930s, and as recently as the 1990s, we can reasonably expect that spinal soft tissues, when injured, can result in both local and referred pain and paresthesiae. From other studies, again both distant and recent, we know that radiating symptoms can be produced by the discs themselves – the so-called discogenic pain.
There is also a growing literature on a condition induced by a process called wind-up wherein it is theorized that the neurons within the spinal cord essentially up-regulate themselves to become more sensitive. The result is that a given tissue irritant or mechanical stimulation results in a heightened perception of pain. This theory of hypersensitivity has some experimental support in animal models, but remains controversial. It also runs on the border of blaming the victim.
References
- Ambroz A, Zucher R, Ambroz C. Strength testing in pain assessment. Pract Pain Management, 2006 Nov / Dec:42-47.
- Agre JC, Magness JL, Hull SZ, et al. Strength testing with a portable dynamometer: reliability for upper and lower extremities. Arch Phys Med Rehabil, Jul 1987;68(7):454-458.
- Croft AC. Pain, impairment, whiplash, and the new AMA Guides: what clinicians need to know. Pract Pain Management. 2014 Jan / Feb:26-33.
- Slipman CW, Plastaras CT, Palmitier RA, Huston CW, Sterenfeld EB. Symptom provocation of fluoroscopically guided cervical nerve root stimulation. Are dynatomal maps identical to dermatomal maps? Spine, 1998;23(20):2235-2242.
- Kawu Ahidjo A, Salami Olayinka AO, Ayokunle O. Klippel-Feil syndrome: a case report. Niger Postgrad Med J, Dec 2010;17(4):320-323.
- Schomacher J, Learman K. Symptom localization tests in the cervical spine: a descriptive study using imaging verification. J Man Manip Ther, Jun 2010;18(2):97-101.
- Kellgren J. On distribution of pain arising from deep somatic structures with charts of segmental pain areas. Clin Sci, 1939;4:35-36.
- Inman V, Saunders J. Referred pain from skeletal structures. J Nerve Ment Dis, 1944;99:660-667.
- Feinstein B, Langton JNK, Jameson RM, Schiller F. Experiments of pain referred from deep somatic tissues. J Bone Joint Surg, 1954;34A(5):981-997.
- Dwyer A, Aprill C, Bogduk N. Cervical zygapophyseal joint pain patterns I: a study in normal volunteers. Spine, 1990;15(6):453-457.
- Aprill C, Dwyer A, Bogduk N. Cervical zygapophyseal joint pain patterns II: a clinical evaluation. Spine, 1990;15(6):458-461.
- Lord SM, Barnsley L, Wallis BJ, Bogduk N. Chronic cervical zygapophysial joint pain after whiplash. A placebo-controlled prevalence study. Spine, 1996 Aug 1;21(15):1737-1744; discussion 1744-1735.
- Travell JG, Simons DG. Travell & Simons' Myofascial Pain and Dysfunction: The Trigger Point Manual. 2nd Edition. Baltimore: Lippincott Williams & Wilkins; 1998.
Click here for previous articles by Arthur Croft, DC, MS, MPH, FACO.





