As the use of dietary supplements has increased, so has concern about product reliability and safety. One reason for such concern is a lack of government oversight into the standards and practices of most supplement manufacturers. While the Food and Drug Administration (FDA) has developed new manufacturing rules for the industry, these regulations have yet to be finalized. Still, little is known about how Americans view the idea of the federal government regulating supplements, and even less is known about their feelings toward product safety and supplement advertising.
To learn more about the attitudes of American adults toward the use and regulation of dietary supplements, a team of investigators from the Harvard School of Public Health and the Henry J. Kaiser Family Foundation conducted an analysis of several recent national opinion surveys. Their analysis, published in the March 26, 2001 issue of the Archives of Internal Medicine,2 found that a majority of people feel supplements are good for overall health and well-being, and believe it is important to have access to them. In addition, the analysis found that although most Americans favor giving the FDA greater regulation over supplement manufacturers, there is still considerable confusion about the government's current role in moderating the supplement industry.
The researchers analyzed data from a half-dozen surveys and opinion polls conducted in the U.S. from 1996 through 1999. Four surveys did not differentiate between the views of supplement users and nonusers; in the other two, respondents were classified as "regular users," "sometimes users" or "nonusers." Most of the information presented in the analysis was derived from the surveys that classified respondents.
Results of the analysis are as follows:
Patient Demographics
- Forty-eight percent of the adults reported taking dietary supplements, vitamins or minerals regularly. About 18% regularly use botanicals, amino acids or synthetic hormones. Fifty-one percent of the men reported using supplements regularly or sometimes, compared to 48% of the women.
- Uninsured Americans were more likely to regularly use supplements than insured Americans. In addition, adults age 45 and older were 16% more likely to regularly use supplements than younger age groups.
- Slightly more than one in six American parents (18%) reported giving dietary supplements to their children.
Beliefs and Attitudes
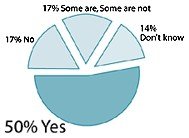
Percentage of American adults who believe supplements are good for health and well-being.
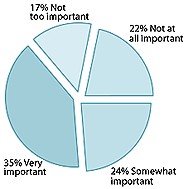
Percentage of American adults who believe access to supplements is:
- Fifty-two percent believed supplements are good for health and well-being. That number increased to 85% for regular users and 62% for sometimes users.
- Many adults believed supplements could be helpful for a variety of conditions, including colds (61%); arthritis (53%); depression (52%) and influenza (49%). Thirty-five percent of the respondents thought supplements could help treat cancer; another 16% thought they could be used to treat AIDS.
- More than half of those surveyed (59%) felt it "very important" or "somewhat important" to have access to supplements, a figure that increased to 91% for regular supplement users.
- In a rather astonishing finding, 72% said they would continue using the supplement they currently use most often even if a government agency said it was ineffective. In a followup survey, respondents were asked what they would do if the FDA specifically indicated the supplement they used most often was ineffective. Sixty-six percent said they would continue taking it.
Patient-Provider Relationships
- In an encouraging sign, more than two-thirds of those who reported taking supplements regularly (70%) said they had shared this information with their physician. However, nearly half of regular users also felt that physicians are "prejudiced" against the use of supplements, and 44% expressed the opinion that their own doctor knows only "a little" or "not much at all" about them.
Product Safety and Truth in Advertising
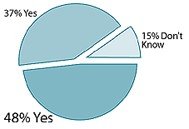
Are dietary supplements adequately tested?
- Only 37% of respondents felt that supplements are adequately tested; nearly half of the respondents (49%) felt that advertising for supplements is "not true." Regular users were more than twice as likely to believe advertising than nonusers.
- Opinions were almost equally divided on the safety of dietary supplements: 47% felt that people are "often or sometimes" harmed by them, while 44% felt they "rarely or never" cause harm.
Government Regulation
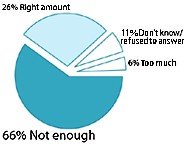
How much regulation is currently in place to make sure that dietary supplements are pure and their doses are consistent?
- The issue of regulation appeared to present the most confusion for respondents. Only 53% were aware that supplements are not regulated by the federal government. The rest either believed supplements are regulated or simply did not know.
- Despite this confusion, an overwhelming majority showed support for more government control over supplements. More than 80% of respondents think the FDA should have the authority to allow new supplements to be sold only if they have been tested first; 80% believe the FDA should have the power to remove supplements from the market if they are shown to be unsafe.
- In addition, many American adults appeared dissatisfied with current regulation levels. Fifty-nine percent of respondents think there aren't enough rules in place to ensure that supplements are not harmful; 60% feel that not enough is being done to make sure supplements are pure and that doses are consistent; and 64% believe there isn't enough regulation to ensure advertising claims are true.
Synopsis
Several noteworthy statistics were revealed in the analysis, primarily the large number of Americans who appear to like (and take) dietary supplements. In fact, many Americans feel so strongly about supplements that they admitted they would continue taking them even if scientific studies proved them to be ineffective.
But this love for supplements is no longer unconditional. Billions of dollars a year are being spent on these products, many of which are not tested for quality or safety. As more money is being poured into the coffers of supplement manufacturers, Americans want to make sure they are getting their money's worth, and that the ingredients listed on a product label are actually in the product. As the researchers noted in their conclusion:
"The growing enthusiasm for dietary supplements does not mean that there is not support for stronger regulation that would require FDA review of the safety of new dietary supplements prior to their sale and that would give the FDA ample authority to remove from the market those products shown to be unsafe."
Before giving the government such power, however, some changes would have to be made in the current typical patient-practitioner relationship. Many of those surveyed "held skeptical views about physicians' and most likely scientists' motivations about dietary supplement issues." The authors concluded Americans would most likely "want clear evidence of a safety hazard" before they would support the removal of a supplement from the market.
Finally, the researchers recommended that practitioners become more familiar with supplements so that their benefits and potential risks can be discussed with patients. They also pushed for more research by government and industry to help ensure product safety.
"There is considerable support for increased research efforts ... to test and evaluate dietary supplements, particularly for safety purposes," the authors remarked. "Given how quickly these products are moving into mainstream use, more research seems warranted."
References
- Gugliona G. Health concerns grow over herbal aids: as industry booms, analysis suggests rising toll in illness and death. Washington Post, March 19, 2000.
- 2Blendon RJ, et al. Americans' views on the use and regulation of dietary supplements. Archives of Internal Medicine March 26, 2001;161:805-810.
Michael Devitt
Senior Associate Editor