Opioids now account for nearly two-thirds of all overdose-related deaths in the U.S. This insidious bane is no respecter of gender, age, race or ethnicity, with nearly all categories experiencing increases. Since 2015, overdose deaths from heroin are up 19.5 percent, prescription opioids 10.6 percent; and in a disturbing rise, death rates from synthetic opioids (such as fentanyl) have risen by more than 100 percent.1
The Neuroscience of Opioid Addiction
Our current understanding is that the rewarding and addictive properties of opiate drugs are mediated by dopamine (DA) neurotransmission in the mesolimbic system. These DA neurons originate in the ventral tegmental area (VTA) of the midbrain and project to the nucleus accumbens (NAc) in the ventral striatum.
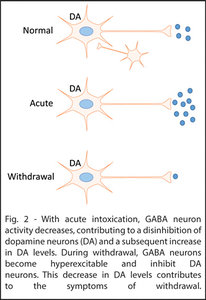
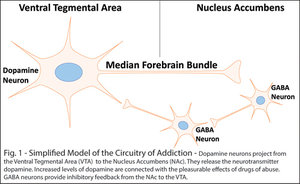 Increased levels of DA are associated with increased reward.2 These levels increase with acute consumption of drugs of abuse, providing a reinforcing stimulus for further use.3-4 During withdrawal, DA neurons become hypoactive while GABA neurons become hyperactive.5-8 Both contribute to a reduction in dopamine neurotransmitter release that is theorized to be the primary driver of withdrawal and relapse.9
Increased levels of DA are associated with increased reward.2 These levels increase with acute consumption of drugs of abuse, providing a reinforcing stimulus for further use.3-4 During withdrawal, DA neurons become hypoactive while GABA neurons become hyperactive.5-8 Both contribute to a reduction in dopamine neurotransmitter release that is theorized to be the primary driver of withdrawal and relapse.9
Opioid Receptors
There are three classic opioid receptor types: kappa (KOR), mu (MOR), and delta (DOR).10-14 Their respective roles in drug abuse disorders are varied, complex and not fully understood. We do know that drugs acting on any of these receptors will produce pain relief through their actions in the spinal cord, brain stem and thalamus.
However, MORs and DORs (the target of prescription opiates and drugs like heroin) produce euphoria through their additional effects on the prefrontal cortex, VTA and NAc.15 Thus, they carry a high risk for dependence and abuse. Conversely, drugs that activate KORs, like the psychotropic salvinorin, produce dysphoria and are not well-suited for use in pain management.16-17
A Physiological Disease or a Psychological Disorder?
Whether addiction is a physiological disease has become hotly contested over the past year. Proponents of addiction as a disease point to the clear changes that occur in the neural circuitry of myriad brain regions as evidence of a progressive disease state. The definition published by the National Institute on Drug Abuse (NIDA) characterizes addiction as a "chronic, relapsing brain disease."
 Others argue that although undoubtedly, brain changes occur in an addicted state, these changes are consistent with changes that occur in any behavioral or habitual activity that is exceedingly rewarding. Perhaps its classification is less important than the approach we take to prevent or treat.
Others argue that although undoubtedly, brain changes occur in an addicted state, these changes are consistent with changes that occur in any behavioral or habitual activity that is exceedingly rewarding. Perhaps its classification is less important than the approach we take to prevent or treat.
I believe the truth in this debate likely lies within both camps. In fact, evidence is clear that a comprehensive approach including, DCs, MDs and PsyDs is far more effective than any one provider trying to treat on their own.
Be wary of approaches that provide the same treatment plan to everyone. The best treatment approaches are individualized and shaped based upon age, race, culture, gender, family, housing, employment, and physical, mental and sexual abuse.
The Spine and Addiction: A Role for Chiropractic?
DCs are uniquely positioned to lead in the battle against opioid addiction for several reasons. First and most obvious, chiropractic care can reduce chronic, intractable pain without the use of narcotic pain medications. Even in situations in which chiropractic care leaves a patient at maximum medical improvement with residual pain, there is a strong argument to be made for utilizing long-term maintenance care as opposed to long-term narcotic painkillers.
This argument has only been strengthened with recent death rates. We needn't shy away from chronic pain maintenance care; it can literally save lives.
Second, there is emerging data suggesting the spine may play a more pivotal role in the circuitry of addiction than previously thought. Data published by Brigham Young University and Daegu Haany University in South Korea show a neurological connection from peripheral mechanoreceptors to the mesolimbic system described above. These studies utilized a variant type of mechano-acupuncture to activate peripheral mechanoreceptors – the same types activated when a patient receives a chiropractic adjustment.
So far, this type of mechanoreceptor-based therapy has been shown to not only involve the opiate pathways in the brain, but also to mitigate drug-seeking behavior in the context of ethanol,18 cocaine19 and methamphetamines.20 In short, when a patient is in withdrawal, stimulation of peripheral mechanoreceptors can calm the part of their brain that's out of control. This calming takes place by inhibition of GABA neurons in the ventral tegmental area and is thought to contribute to the noted decrease in drug-seeking behavior.
Further, the pathways underlying these effects are being elucidated and are integrally related to the science of chiropractic. They act, in large part, through activation of the dorsal column medial lemniscal pathway (DCML).21 This pathway should cause every chiropractor's spine to tingle. It is central to every adjustment given to every patient since D.D. met Harvey.
Regardless of technique, mechanoreceptors function as intermediaries between the doctor and the patient's brain, and the DCML is the neurological pathway that provides us access. Research into this connection is ongoing, with several more papers to be published in the next six months that will shed further light on the power the adjustment has to influence brain function.
The Bottom Line
Chiropractors have an increasingly justified case to be more involved in solving the opioid crisis. Though pharmacological treatments for addiction should not be overlooked, logic dictates that only using an addictive chemical to treat the effects of an addiction to a chemical would not likely produce the highest rates of success.
We have a moral duty to provide the best care possible to our patients, and the data is suggestive that an all-of-the-above approach provides the highest probability of a good outcome. All of the above includes us!
References
- Centers for Disease Control and Prevention. C.f.D.C.a.P., Multiple Cause of Death. CDC WONDER Online Database, 2017.
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res, 2008;14(2-3):169-83.
- Carboni E, et al. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neurosci, 1989;28(3):653-61.
- Yoshimoto K, et al. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol, 1992;9(1):17-22.
- Koeltzow TE, White FJ. Behavioral depression during cocaine withdrawal is associated with decreased spontaneous activity of ventral tegmental area dopamine neurons. Behav Neurosci, 2003;117(4):860-5.
- Karkhanis AN, et al. Switch from excitatory to inhibitory actions of ethanol on dopamine levels after chronic exposure: Role of kappa opioid receptors. Neuropharmacol, 2016;110:190-197.
- Maisonneuve LM, Ho A, Kreek MJ. Chronic administration of a cocaine "binge" alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther, 1995;272(2):652-7.
- Rose JH, et al. Supersensitive kappa opioid receptors promotes ethanol withdrawal-related behaviors and reduce dopamine signaling in the nucleus accumbens. Int J Neuropsychopharmacol, 2016;19(5).
- Lyness WH, Smith FL. Influence of dopaminergic and serotonergic neurons on intravenous ethanol self-administration in the rat. Pharmacol Biochem Behav, 1992;42(1):187-92.
- Attali B, Saya D, Vogel Z. Kappa-opiate agonists inhibit adenylate cyclase and produce heterologous desensitization in rat spinal cord. J Neurochem, 1989;52(2):360-9.
- Konkoy CS, Childers SR. Dynorphin-selective inhibition of adenylyl cyclase in guinea pig cerebellum membranes. Mol Pharmacol, 1989;36(4):627-33.
- Prather PL, et al. Properties of a kappa-opioid receptor expressed in CHO cells: interaction with multiple G-proteins is not specific for any individual G alpha subunit and is similar to that of other opioid receptors. Brain Res Mol Brain Res, 1995;29(2):336-46.
- Tallent M, et al. The cloned kappa opioid receptor couples to an N-type calcium current in undifferentiated PC-12 cells. Neurosci, 1994;63(4):1033-40.
- Henry DJ, et al. Kappa-opioid receptors couple to inwardly rectifying potassium channels when coexpressed by Xenopus oocytes. Mol Pharmacol, 1995;47(3):551-7.
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci, 1994;14(4):1978-84.
- Pfeiffer A, et al. Psychotomimesis mediated by kappa opiate receptors. Science, 1986;233(4765):774-6.
- Roth BL, et al. Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci, 2002;99(18):11934-9.
- Yang CH, et al. Acupuncture inhibits GABA neuron activity in the ventral tegmental area and reduces ethanol self-administration. Alcohol Clin Exp Res, 2010;34(12):2137-46.
- Yoon SS, et al. Effects of acupuncture on stress-induced relapse to cocaine-seeking in rats. Psychopharmacol, 2012;222(2):303-11.
- Kim NJ, et al. Acupuncture inhibition of methamphetamine-induced behaviors, dopamine release and hyperthermia in the nucleus accumbens: mediation of group II mGluR. Addict Biol, 2018.
- Chang S, et al. Spinal pathways involved in somatosensory inhibition of the psychomotor actions of cocaine. Sci Rep, 2017;7(1):5359.
Dr. Kyl Bills is a PhD candidate in the Interdepartmental Neuroscience Program at Brigham Young University, focusing on addiction research in the Steffensen Addiction Laboratory. He is a graduate of BYU and Parker University.




