One of the most exciting things about manual methods such as Graston, active release, fascial manipulation, friction massage and others is that mechanical loading stimulates the proliferation of fibroblasts.
The composition of the extracellular matrix determines the physical properties of connective tissues – whether they become tendons, ligaments, etc. So, the collagen, glycosaminoglycans, glycoproteins, reticular and elastic fibers all found in the ECM are made by fibroblasts. Collagen is considered the most important ECM component since fibrillar collagen takes most of the tensile loading. Can you imagine that we help create these changes by frictioning tissues?1-2
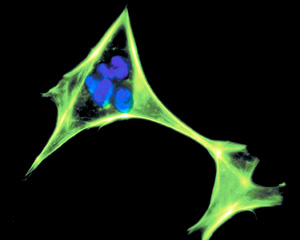 Microscopic view of a human fibroblast cell, stained against DNA and actin.
Fibroblastic proliferation and degradation is a normal occurrence in everyday mechanical loading such as walking, running or most forms of movement. Even mechanical loading during rest and sleep relates to connective tissue function. Four basic types of loads are usually considered: tension (tensile forces-fibroblasts), compression (chondrocytes), fluid shear (vascular endothelial cells) and torsional shear.3 Collagen synthesis in the patellar tendon increases by nearly 100 percent as a result of just a single bout of acute exercise, and the effect is still evident three days later.
Microscopic view of a human fibroblast cell, stained against DNA and actin.
Fibroblastic proliferation and degradation is a normal occurrence in everyday mechanical loading such as walking, running or most forms of movement. Even mechanical loading during rest and sleep relates to connective tissue function. Four basic types of loads are usually considered: tension (tensile forces-fibroblasts), compression (chondrocytes), fluid shear (vascular endothelial cells) and torsional shear.3 Collagen synthesis in the patellar tendon increases by nearly 100 percent as a result of just a single bout of acute exercise, and the effect is still evident three days later.
In the initial training period, collagen turnover in tendons (i.e., the balance between synthesis and degradation) is actually increased and there is a net loss of collagen. This enables a tendon to restructure and adapt to an increased loading pattern. It is not until training progresses that there is a net gain in collagen synthesis.4 Could deep friction massage be considered a localized exercise?
Fibroblasts also play an essential role in wound healing. After the initial injury to connective tissue and blood vessels, growth factors cause fibroblasts to enter the wound, increase in number and start synthesizing new collagen, creating new granulation tissue and assisting in remodeling. The extracellular matrix of granulation tissue is created and modified by fibroblasts. At first, the fibroblasts produce type III collagen, a weaker form of the structural protein; and later produce the stronger, long-stranded type I collagen that appears in the scar tissue.
A scar is collagen deposited by fibroblasts during repair. Collagens I to III represent 80-90 percent of the collagen in the body,5 with the majority being type I. Fascia is a type I form of collagen. Graston Technique and fascial manipulation both work on the premise that small amounts of trauma to an area initiate an inflammatory process, triggering a healing cascade by enhancing the proliferative invasion of blood, nutrients, and fibroblasts to the region, which results in collagen deposition and maturation.6
One of the main arguments for the use of mechanical load on conditions such as tendinosis is that "fibroblasts in tendinosis are extremely active metabolically and there is a great capacity for the production of collagen. A fibroblast-driven process would be expected to integrate old and new collagen in order to contribute to the final stability of the matrix."7 Fibroblasts can also convert into myofibroblasts necessary in wound strengthening by extracellular collagen fiber deposition, and wound contraction for closing wounds since myofibroblasts express -smooth-muscle actin. Upon resolution of the injury, these activated fibroblasts (myofibroblasts) undergo apoptosis (programmed cell death).
Tendons that undergo high rates of stretching may be more susceptible to inflammation and eventual degeneration due to the stretching of fibroblasts. Cyclic stretching of fibroblasts, and especially increasing the frequency of the stretching increases the production of pro-inflammatory cyclooxygenase enzyme (COX-1, COX-2) and prostaglandin-E2. COX-1 and COX-2 convert arachidonic acid into prostaglandins. So, overstimulation of the fibroblasts may be responsible for repetitive-motion problems. Stretching also may cause an alignment of the tendon fibroblasts.8
Recent studies have shown how eccentric exercises may be more beneficial than concentric exercises regarding the rehabilitation of muscles and tendons.9-10 There is reason to believe that the effect of the load pattern of eccentric exercise creates greater stimulation of fibroblasts, increasing collagen synthesis and thereby stimulating the healing of the injured tissue.11
Finally, Langevin12 poses the theory that connective tissue, especially the fibroblasts are part of a whole body cell to cell communication signaling network. She states that fibroblasts exhibit active cytoskeletal responses within minutes of tissue lengthening. "Analogous cell-to-cell signaling involving calcium and/or ATP may exist within connective tissue and may be accompanied by active tissue contraction or relaxation. One can envisage the whole-body web of connective tissue involved in a dynamic, body-wide pattern of cellular activity fluctuating over seconds to minutes reflecting all externally and internally generated mechanical forces acting upon the body."
Author's note: The International Myofascial Pain Conference will be held Sept. 3-4; the 2nd Annual Myofascial Pain Conference takes place Oct. 1-2. For more information, visit www.fascialconference.com.
References
- Hammer W. The effect of mechanical load on degenerated soft tissue. Journal of Bodywork and Movement Therapies, 2008;12:246–256.
- Loghmani MT, Warden SJ. Instrument-assisted cross-fiber massage accelerates knee ligament healing. Journal of Orthopaedic & Sports Physical Therapy, 2009 Jul;39(7):506-514.
- Eastwood M, McGrouther, Brown RA. Fibroblast responses to mechanical forces. Proc Instn Mech Engrs, 1998;212(Part H):85-92.
- Benjamin M, Kaiser E, Milz S. Structure-function relationships in tendons: a review. J Anat, 2008:;212(3):211-28.
- Lundon K. Orthopedic Rehabilitation Science. Butterworth-Heinemann, NY, 2003:5-32.
- Carey-Loghmani MT, Hammer WI. Graston Technique. In: Hammer WI. Functional Soft-Tissue Examination and Treatment by Manual Methods, 3rd Edition. Sudbury, MA, Jones & Bartlett, 2007:589-625.
- Kraushaar BS, Nirschl RP. Tendinosis of the elbow (tennis elbow). Journal of Bone and Joint Surgery, 1999;81-A(2):259–276.
- Wang J, Li Z, Yang G, Khan M. Repetitively stretched tendon fibroblasts produce inflammatory mediators. Clinical Orthopaedics and Related Research,2004;422:243-250.
- Honsson P, Alfredson H. Superior results with eccentric compared to concentric quadriceps training in patients with jumper's knee: a prospective randomized study. British Journal of Sports Medicine, 2005;39(11):847-50.
- Bernhardsson S, Klintberg IH, Wendt GK. Evaluation of an exercise concept focusing on eccentric strength training of the rotator cuff for patients with subacromial impingement syndrome. Clinical Rehabilitation, 2011;25:69-78.
- Jeer M, Langberg H, Heinemeier K, et al. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scandinavian Journal of Medicine & Science in Sports, 2009;19:500-510.
- Langevin HM. Connective tissue: a body-wide signaling network? Medical Hypotheses,2006;66:1074-1077.
Click here for previous articles by Warren Hammer, MS, DC, DABCO.





